Monoamine Re-uptake Inhibitors Mechanism of Action in Pain PathwayDuring this visit, you apply knowledge of the physiological and genetic mechanisms of pain and pain susceptibility, and mechanisms that modulate acute and chronic pain susceptibility in case presentations, patient simulations, and care of patients.
Your patient reports jaw clicking with and without pain, and frequent headaches for 3 months.
Biopsy
After biopsy by palate approach, the patient is treated with:
- cyclophosphamide, doxorubicin, vincristine, prednisone
- radiation
- A G-tube is placed due to risk of malnutrition from tumor site and treatment with chemotherapy and radiation to orofacial region.
What medications and bio behavioral interventions would YOU recommend for symptom management?
In the following pages, we’ll take a look at some of those that are more commonly prescribed. Think about why these might have been prescribed or tried and if there is any rationale based on the neurophysiology and genetics of pain.
Is there anything else that you would have prescribed or recommended?
Medications
Your patient is receiving the following medications for symptom management. What is the indication for these pharmacologic treatments?
- Hydromorphine (Diloudid) 4 mg every 4 hours PRN pain
- Peridex 15 ml PO BID
- Lansoprazole (prevacid) 30 mg PO daily
- Ondansetron (Zofran) 8 mg ODT every 8 hours PRN nausea or anxiety
- Lorazepam (ativan) 0.5 mg PO every 6 hours PRD nausea or anxiety
- Senna (Senokot) 2 tabs PO 1 to 2 time daily to achieve upt to 2 BMs/day
- Polyethylene glycol (Miralax) 17 grams 1 to 2 time daily to achieve up to 2 BMs/day
What other medication treatments might you consider?
Opioid Analgesics
Opium, the latex of unripe poppy seeds, is among the oldest recorded medications in use by humans; possibly used as early as 3500 BC.
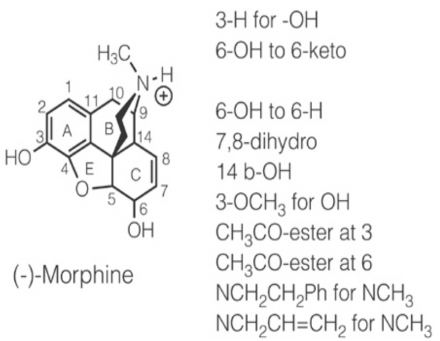
Opiates are naturally occurring alkaloids derived from opium (e.g., morphine, codeine). Morphine was first isolated in 1803 and was one of the first structures to be structurally modified.
Opioids are opium-like synthetic substances (e.g. oxycodone, fentanyl, hydromorphone, methadone). Opioids induce analgesia (decrease pain) without causing narcosis, if used appropriately.
Opioids are also called narcotics – but the term narcotic can be misleading.
Narcotic means agents causing sleep or loss of consciousness (narcosis)
Narcotic has also become associated with addictive properties of opioids
It is also a legal term that includes a wide range of sedating and potentially abused substances, not only opioids.
Opioids Mechanism of Action
Opioid receptors are distributed throughout the brain, spinal cord, peripheral nervous system, skin, and joints. Opioids have multiple sites of action, including: medulla, spinal cord, spinal trigeminal nucleus, and periaquaductal grey (PAG) which is an integration modulation site from peripheral nerves to the central neuraxis. In the brain, opioids activate descending pain inhibitors.
Opioids mimic the actions of endogenous opioid eptides by interacting with opioid receptors designated mu (μ), kappa (κ), and sigma (σ). Opioids provide pain relief by:
- Decreasing pre-synaptic release of excitatory neurotransmitters
- Decreasing post-synaptic neuronal excitability
- Promoting descending inhibition
Opioid analgesics are characterized by their pharmacologic differences which are derived from their complex interactions with three opioid receptors, mu (μ), kappa (κ), and delta (δ) with evidence for subtypes.
A 4th opioid receptor has been identified but does not bind classical opioid agonists or antagonists (considered an orphan receptor)
The μ receptor is the most important for analgesia and opioid- induced respiratory depression. There are at least two subtypes; μ1 probably mediates respiratory depression, bradycardia, and physical dependence.
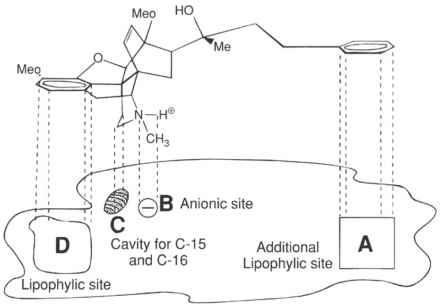
This diagram illustrates the opioid receptor binding model. It shows the binding sites and the chemical structure of morphine.
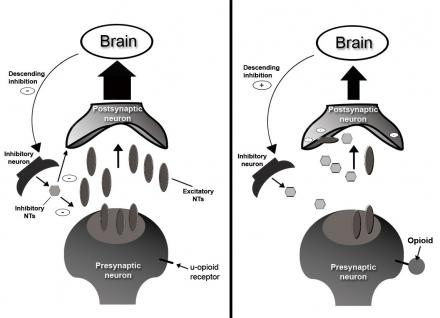
The opioid receptors are coupled to G1 proteins and the actions of the opioids are mainly inhibitory. They close N-type voltage-operated calcium channels and open calcium-dependent inwardly-rectifying potassium channels.
This results in hyperpolarization and a reduction in neuronal excitability. They also decrease intracellular cAMP, which modulates the release of nociceptive neurotransmitters (e.g. substance P)
This second diagram shows how these would fit together for morphine to bind at the receptor site.
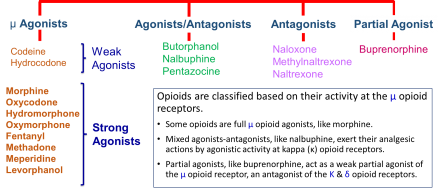
μ Opioid Receptor Selective Compounds
Notice the differences in chemical structure of μ opiates and how these structures lead to variability in efficacy.
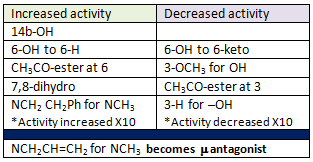
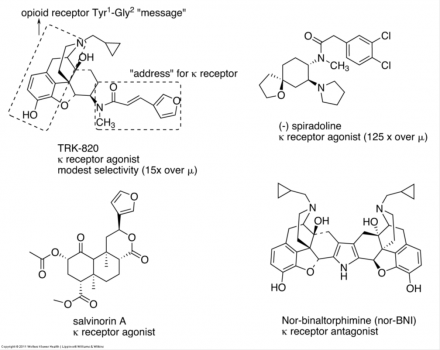
κ Opioid Receptor Selective Compounds
κ opiates also have varying efficacy based on differences in their chemical structure as illustrated here.
Opioid Activity
The efficacy of analgesia will vary between individuals. The true determinant of treating an individual with different opioids is dependent on the variability and adverse effects.
Desired Effect: Analgesia
Adverse Effects:
- Reduced GI motility/Constipation
- Nausea/Vomiting
- Sedation
- Dyspnea/Respiratory depression
- Euphoria/dysphoria
- Pruritis
Opioid Variability
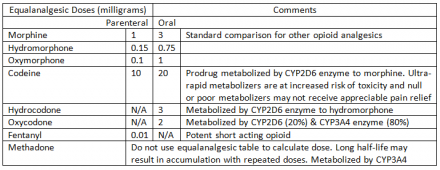
Opioid variability must be translated for patient dosing in clinical practice to provide approximate equianalgesia.
It was widely believed that there was no maximum opioid dose, and that increased doses led to increased analgesia if the patient was tolerant enough to withstand opioid side-effects, like respiratory depression.
However, lack of pain reduction at higher opioid doses and increased deaths and addiction at high doses strongly support a ceiling of analgesia from opioids, as well.
Pharmacogenetics- Morphine
In general, current strategies for drug dosing are based on the assumption that a patient is an extensive metabolizer (EM). Persons who are EMs usually comprise the largest proportion of the population.
For Poor Metabolizers (PM), the targeted medication is metabolized slowly or not at all, which increases the risk of side effects, some which might be lethal. PMs more than likely require lower doses of the target medication compared with standard EMs.
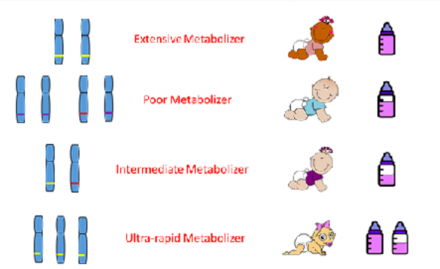
Intermediate Metabolizers (IM) break down the targeted medication at a slower rate than EMs, possibly requiring a lower than normal dose of the medication.
Ultra-rapid Metabolizers (UM) metabolize a targeted medication rapidly, resulting in decreased bioavailability of the drug. This can result in a poor therapeutic response, possibly mandating a dose higher than the standard for that medication.
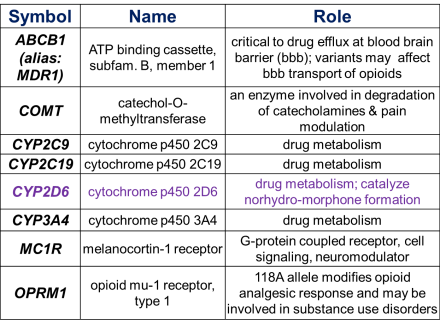
Select Genes Relevant to Analgesic Metabolism
Gene variants alter analgesic metabolism. There is a large interethnic variability in the proportion of PM, IM, and UM.
For example:
Among whites, UMs of the CYP2D6 enzyme comprise 3% to 5% of the population, whereas EMs comprise 70% to 80%, IMs comprise 10% to 17%, and 5% to 10% are PMs.
Among Chinese, PMs of the CYPD26 enzyme account for approximately 1% of the population, whereas 0% to 19% of African-Americans are PMs.
Additionally, 1% to 2% of Swedish whites and 16% of black Ethiopians are the UM phenotype.
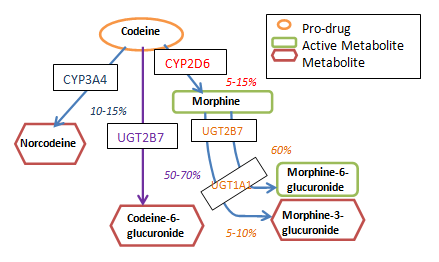
Pharmacogenetics – Morphine & Codeine Metabolism
Morphine and Codeine Metabolism
Opiate metabolism may explain individual patient differences in analgesic efficacy.
Codeine is metabolized to morphine.
Mechanism of Action of Anti-inflammatory Drugs
Pro-drugs
Codeine is a pro-drug; it has no analgesic properties until it is metabolized to morphine.
Individuals who state codeine does not relieve their pain may not be able to metabolize codeine to morphine.
Anticonvulsants Mechanism of Action in Pain Pathway
Anticonvulsants, also known as α2-δ ligands, bind to α2-δ subunit of voltage-dependent calcium channels within neuronal membranes.
They are indicated for neuropathic pain and commonly used agents are gabapentin and pregabalin.
Proposed analgesia mechanism:
- Inhibit release of excitatory neurotransmitter by pre-synaptic neurons
- Activate descending inhibitory pathways to increase spinal norepinephrine concentration
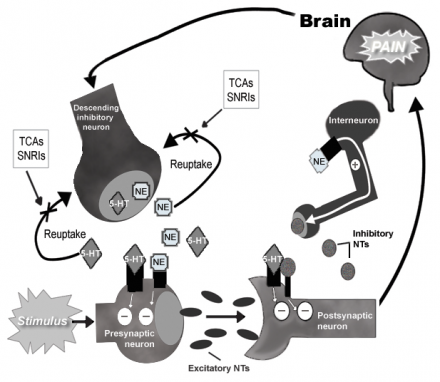
Monoamine Re-uptake Inhibitors Mechanism of Action in Pain Pathway
Monoamine re-uptake inhibitors are indicated for neuropathic pain and include:
- Tricyclic antidepressants:
- Amitriptyline, nortriptyline, desipramine, imipramine
- Serotonin and norepinephrine reuptake inhibitors (SNRIs):
- Duloxetine, minalcipran
Provides analgesia by:
- Increasing synaptic concentration of norepinephrine (NE), and/or serotonin (5-HT)
- Inhibit release of excitatory neurotransmitter (NT) by pre-synaptic neurons
- Reducing the excitation of post synaptic neurons
- Promoting release of other inhibitory NTs
Local Anesthetics Mechanism of Action in Pain Pathway
Local anesthetics provide pain relief by blocking neuronal membrane sodium channels within tissues at the site of application. This results in:
- Inhibiting afferent neuronal excitability
- Inhibiting propagation of nociceptive input via action potentials
- They are indicated for acute, post-operative, and neuropathic pain.
This group includes:
- Lidocaine 5% patch
- Bupivacaine
- Ropivicaine
Case Continues
Consider the following bio-behavioral interventions for symptom management:
- Guided imagery training
- Counseling
- Biofeedback
- Massage for nausea management
- Aromatherapy
- Others patient has found effective in the past
- Is there anything else you would have prescribed or recommended?
Non-Pharmacologic Treatment
Non-pharmacologic or biobehavioral treatments work by a variety of mechanisms and sites to relieve pain. Efficacy of analgesics and non-pharmacologic interventions are evaluated based on comparisons of sample norms; but there is tremendous variability in patients’ treatment responses.
Your patient reports jaw clicking with and without pain, and frequent headaches for three months. Treatments have included application of ice, teeth filing, traction, and corrective lenses for reading. The patient reports ice helped to some extent, but nothing else seemed to decrease the frequency or severity of symptoms.
- How do non-pharmacologic treatments for pain work?
- Should a trial of transcutaneous electrical nerve stimulation be considered?
During this visit, the mechanism of action for ice and Transcutaneous Electrical Nerve Stimulation will be outlined for a better understanding of their potential effectiveness for reducing or relieving your patient’s pain.
Cold Therapy
Cold therapy refers to the superficial application of cold to the surface of the skin, with or without compression and with or without a mechanical recirculating device to maintain cold temperatures.
Localized cold therapy has commonly been used in acute pain, including trauma and postoperative pain. The potential benefits at the site of injury are thought to be related to reductions in tissue temperature, resulting in reduced edema and local analgesia.
Trials of cold therapy, however, have been inconsistent and frequently found no differences compared to no cold therapy for pain, edema or analgesic use. These findings suggest that cold has no long lasting benefits; rather it provides temporary numbness at the site of pain.
The mechanisms beyond pain relief following brief applications of cold have not been elucidated.
With more prolonged application (e.g. 10 minutes or more over the jaw), cold results in slowing of nerve conduction velocity, particularly in unmyelinated (C) and thinly myelinated (A-delta) primary afferent nerves.
Pain signaling carried along these afferents is slowed, limiting transmission to the dorsal horn, or spinal trigeminal nucleus for orofacial pain, and diminishing pain sensation.
TENS – Transcutaneous Electrical Nerve Stimulation
The mechanism of action for transcutaneous electrical nerve stimulation (TENS) is thought to be through stimulation of large-diameter cutaneous nerve fibers to inhibit nociceptive responses along the descending pathway. Low and high frequency TENS also release endogenous opioids.
Pain Perception and Control
Pain is modulated through the stimulation of peripheral primary afferent nerves through the delivery of electrical current across the skin (transcutaneous)
Stimulation of Innervated Muscle
Electrical current can also be used to stimulate muscle contraction through electrically induced depolarization of the alpha motor neuron. Often termed neuromuscular electrical stimulation (NMES), the technique still induces nerve depolarization via a transcutaneous electrical current and is thus a special form of TENS.
Gate Control Theory
Mechanism through which neural impulses ascend to the dorsal horn (spinal trigeminal nucleus for orofacial pain), affecting pain perception.
Stimulation of A-beta primary afferents stimulates the release of enkephalin, blocking synaptic transmission of pain signals.
This ascending influence was originally proposed in the Gate Control Theory section of this course (see the Redflags Visit to learn more about models of pain).
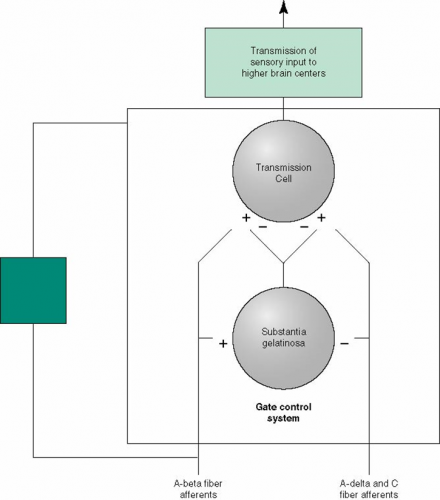
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
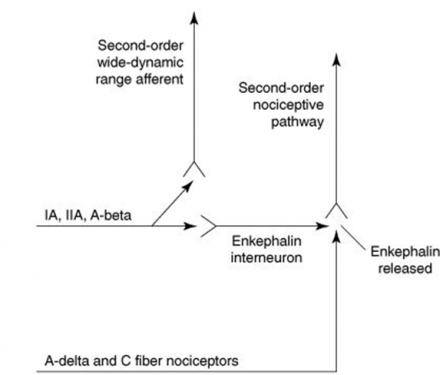
Descending Influence Model of Pain Control
Stimulation of rhythmic muscle contractions or C primary afferents (acupuncture-like TENS) is believed to activate descending pain modulation mechanisms of large-diameter cutaneous nerve fibers to inhibit nociception.
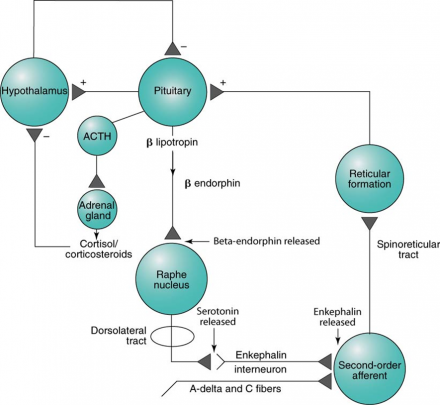
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
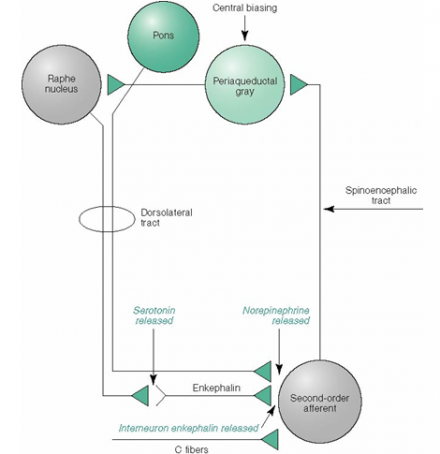
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
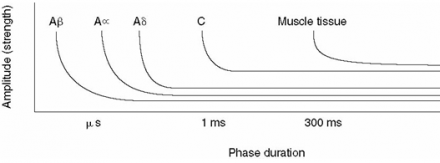
Depolarization and Strength-Duration Curves of Tissues
Pulses of electrical energy cause depolarization of peripheral nerves.
Stimulus A is insufficient to depolarize the nerve, while stimulus B overcomes resting capacitance and results in depolarization.
Less energy is needed to depolarize large diameter (e.g. A-beta afferent) fibers, thus these can be selectively stimulated.
Stimulation results in the release of encephalin in the dorsal horn or trigeminal. Enkephalin inhibits the release of pain promoting transmitter substances (e.g. substance P) and thus blocks the transmission of the pain signal.
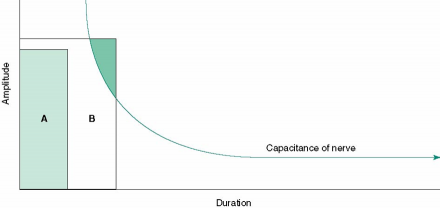
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
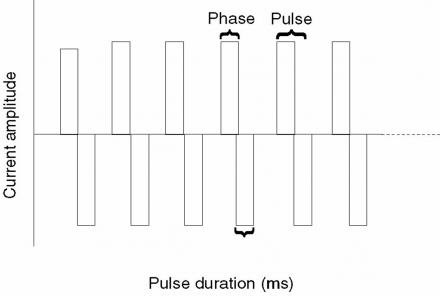
Types of Current
Electrical currents generated by the TENS device are pulsatile.
- monophasic (current flows in one direction)
- biphasic (current flows first from one direction, then the other)
- polyphasic (time- modulated AC)
This depiction of an Interval impulse is an example of pulsatile biphasic current.
*Image used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
Waveform is not a determinant of which afferent phenotypes are recruited.
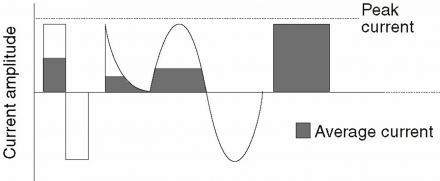
Key parameters
- Amplitude - strength of the current delivered
- Phase duration - duration of the pulse (measured in microseconds)
- Frequency – number of times per second depolarization occurs
*Image used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
Diagnosis
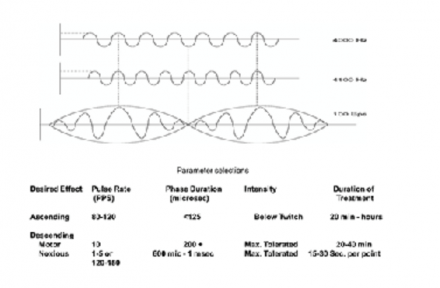
For this patient, a trial of TENS for pain relief via A-beta afferent stimulation can be considered.
Stimulation of descending modulation pathways requires adjusting the TENS unti parameters. Facial stimulation may be poorly tolerated by some patients.
*Images used by permission: Denegar et al. (2016) Therapeutic Modalities for Musculoskeletal Injuries.Health Kinetics
Clinical Decisions for Pain Control Using TENS
- Low risk (do not place electrode over baroreceptors of the carotid artery or eyes; pregnancy and cardiac pacemaker may contra-indicate use)
- Low cost – devices can be purchased for under $100
- Patient-controlled relief
- Limited evidence of effectiveness in patients with maxofacial and neurogenic pain - however, full resolution of pain has been reported in some patients


