Treatment Options for Poor Sleep
- sleep hygiene
- sleep medications
- Behavioral approaches (CBT, hypnosis, relaxation, meditation, etc.)
- treatment of parasomnias
- Treatment of sleep apnea
Sleep Disorders
What are the guidelines for good sleep hygiene?
How does poor sleep influence chronic pain?
What is the epidemiology of sleep problems? What comorbidities are associated with obstructive sleep apnea (OSA)? See Gotsopoulos H 2004, Rao M 2010, Marin 2012. Bratton 2015, Baglioni C 2011 Pfillips CL 2013
How do you determine whether your patient has a sleep disorder? (objective and subjective measures, PSQI, Stop Bang, ESS tools)
How does one define the severity of, diagnose , and treat obstructive sleep apnea (OSA)? Bianchi 2009, Sutherland K 2014 & 2015, Ramar K 2015
How do restless leg syndrome (RLS) , periodic limb movements (PLMS), and bruxism affect sleep quality? Dickoff DJ. RLS-Migraine-Bruxism-triad?Sleep review .com/2015
Good Sleep Practice/Hygiene
- Establish a consistent pre-sleep routine
- Limit daytime naps
- Minimize light, bedroom noise, and extreme temperatures
- Avoid large meals and alcoholic beverages before bedtime
- Avoid strenuous exercise within 2-3 hours of bedtime
- Avoid caffeine or nicotine-type stimulants before bedtime
Poor Sleep and Chronic Low Back Pain
How is poor sleep a factor in chronic low back pain?
- Many studies report that > 50% of patients with chronic low back pain (CLBP) suffer from sleep disturbances and poor sleep quality
- Patients with CLBP spend < 5% of the time in stage 3 and 4 sleep (deep sleep) versus 20-25% of the time for patients without pain
- Depression is associated with sleep disorders and chronic low back pain
- Sleep and pain are cyclical and bidirectional; decreased sleep leads to increased pain and increased pain leads to decreased sleep
- In summary, decreased sleep and poor sleep quality leads to:
- Increased pain and hyperalgesia
- Decreased quality of life
- Decreased coping mechanisms
- Increased rate of hospitalizations
- And more…
Sleepless in America
- 67% of adults report more than 1 sleep disorder symptom
- 7% have been told they have a sleep disorder by a M.D.
- 32% of Americans sleep 6 hours / night or less
- 23% of adults fell asleep while driving last year
Snoring & Obstructive Sleep Apnea (OSA) in the U.S.
- Approximately 40% of adults over 40 years old snore (about 100 million Americans)
- 4% of men and 2% of women have signs and symptoms of OSA (about 12 million Americans)
- Obstructive Sleep Apnea (OSA) is as prevalent as diabetes or asthma.
Dangers of Snoring
How Loud is Snoring Compared to Other Noises?
- Jackhammer: 85 decibels
- Lawnmower: 95 decibels
- Airplane: 118 decibels
- Loudest recorded snoring: 87 decibels
Risks
- Increased motor vehicle accidents:
- > 3 x more than general population
- > 7 x greater risk of multiple MVAs
- Effect on sleep partner
- Indication of sleep apnea and it’s co-morbidities?
Snoring Effects on Sleep Partner
Mayo clinic study:
- Snorer partially awakened 27 times/hour while spouses are partially awakened 21 times/hour
- Spouses had increased pain complaints
- Spouses had a reduced quality of life, which was improved significantly when snoring was treated
Comorbidities with Sleep Disorders
The following can be associated with sleep disorders:
- The presence of parasomnias such as restless leg syndrome (RLS), periodic limb movements (PLMS), and bruxism (teeth grinding) can identify the patient for an increased hazard from OSA.
- Depression and anxiety
- Hypertension and cardiovascular problems
- Weight gain
RLS = restless leg syndrome
PLMS = periodic limb movement syndrome
Bruxsim = Diurnal or nocturnal parafunctional activity including clenching, bracing, gnashing, and grinding of the teeth
Determining Sleep Disorder
How do you determine whether your patient has a sleep disorder, in particular obstructive sleep apnea (OSA)?
Subjective measures:
- Patient self-report using validated questionnaires (e.g. Pittsburgh Sleep Quality Index (PSQI), the Medical Outcomes Study Sleep Scale, etc.)
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval. Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of scores for these seven components yields one global score. Click here for an example of the PSQI.
Epworth Sleepiness Scale (ESS)

- ESS is only a tool, not a definitive diagnostic test
- Maximum score= 24
- In general, if a patient snores and:
- if ESS total is < 8, OSA is less likely
- if ESS total is 9-11, OSA is uncertain
- if ESS total is > 12, OSA is more likely
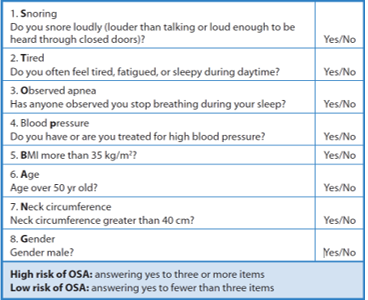
STOP-BANG Questionnaire
Objective Measures for Assessing Sleep Disturbances/OSA
What are objective measures for assessing sleep disturbances/OSA?
Objective measures (do not always correlate with subjective reports):
- Actigraphy:
- assesses movement during sleep and quantification of sleep time, sleep efficiency, and sleep disturbance
- objectively distinguishes between sleep and wakefulness using activity counts as a proxy measure of sleep
- less expensive and intrusive than PSG, reasonably high agreement with PSG
- Polysomnography (PSG)
- Standard objective measure with simultaneous assessment of:
- Electroencephalography (EEG, brain activity)
- electrooculography (EOG, eye movement)
- electromyography (EMG, muscle activity)
- Portable/home sleep testing (“unattended” PSG)
- May include any combination of the following measures:
- Nasal and oral air flow
- Respiratory effort (by elastic belt bands across chest and abdomen)
- Body position
- End-tidal CO2 monitoring (capnography)
- Oxygen saturation (pulse oximetry)
- Heart rate and rhythm (electrocardiography (ECG))
- May include any combination of the following measures:
Sleep Study Diagnosis:
- Test includes: oximetry, heart rate, airflow (nasal pressure and thermister), chest movement, abdominal movement, snoring, body position, nasal pressure, EEG, EOG (electrooculogram), neck EMG, leg EMG = CL 1 test
- Provides detailed report with number of apneas (obstructive, central, mixed), hypopneas, AHI, arousals and cause of arousals, awakenings, response to PAP (for split or titration studies), duration and severity of snoring, sleep disorder diagnosis, and recommendations for therapy
- Collop NA; Anderson WM; Boehlecke B; Claman D; Goldberg R; Gottlieb DJ; Hudgel D; Sateia M; Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007 ;3(7):737-747.
Example of a Portable PSG
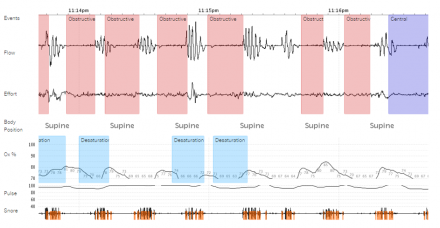
Portable/Home Sleep Apnea Test
Good for:
- High clinical suspicion for severe OSA without significant other sleep disorders or other medical co-morbidities
Not good for:
- Low suspicion for OSA
- Severe lung disease
- Congestive Heart Failure
- Neuromuscular disease or suspected hypoventilation syndrome
- Suspected other sleep disorder: insomnia, periodic limb movement disorder, restless legs, RBD, narcolepsy
Home sleep test (HST) is only useful if it confirms your suspicion of OSA – it is useless if it doesn’t confirm Obstructive Sleep Apnea (OSA)!
- Does not assess for any other sleep disorder!!!
- Non-diagnostic HST should be followed by a polysomnogram!
(Courtesy of Dr. Christopher Humphries, Salem MA.)
Full Night Attended Diagnostic Polysomnogram (PSG)
- Measures physiologic activity during sleep
- EEG and EOG: Sleep stages (N1, N2, N3, REM, wake) and patterns, arousals
- Airflow via nasal pressure and oronasal thermistor
- Movement of chest and abdomen via plethysmography
- Snoring via microphone
- Continuous oximetry
- Single lead ECG
- Leg and chin EMG
- Gold standard diagnostic sleep testing for OSA, CSA, PLMD required for narcolepsy, idiopathic hypersomnia
Test includes: oximetry, heart rate, airflow (nasal pressure and thermistor), chest movement, abdominal movement, snoring, body position, nasal pressure, EEG, EOG (electrooculogram), neck EMG, leg EMG = CL 1 test
Provides detailed report with number of apneas (obstructive, central, mixed), hypopneas, AHI, arousals and cause of arousals, awakenings, response to PAP (for split or titration studies), duration and severity of snoring, sleep disorder diagnosis, and recommendations for therapy
OSA = obstructive sleep apnea
CSA = central sleep apnea
PLMD = periodic limb movement disorder
EEG:Electroencephalography
EOG:Electrooculography
EMG:Electromyograph
ECC-Electrocardiogram
PAP= Positive airway pressure
AHI=apnea/hypoapnea index
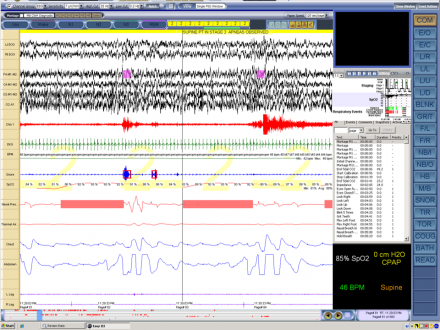
Polysomnogram (PSG) Showing Apneic Events
This is a portion of a PSG (polysomnogram) showing apneic events during which the blood oxygen level drops to 85% This represents a 120 second epoch demonstrating stage 2 sleep with obstructive apneas: see reduced airflow and hypoxemia marking these apneas.
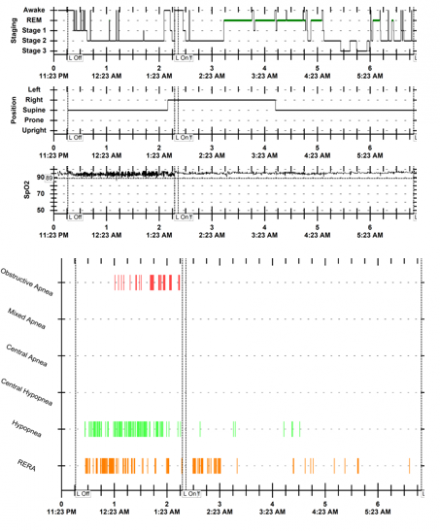
Example of Polysomnogram Data
This slide shows an example of PSG data. These are summary graphs of a split night polysomnogram demonstrating mixture of apneas and hypopneas with hypoxemia during the diagnostic phase, which are then controlled with CPAP during the titration phase of the study.
RERA = respiratory effort related arousals
Mr. Wakefield's Polysomnogram Results
Evaluating Mr. Wakefield’s sleep and the role it plays in his chronic pain:
results of the polysomnogram.
Mr. Wakefield’s daytime somnolence requires a polysomnogram to evaluate for sleep apnea. A review of the PSG report reveals an AHI of 18, an RDI of 30, and an oxygen nadir of 84% with 9 minutes during which his blood oxygen level was below 90%. The study also indicates that he has bruxing activity and clenches his teeth during sleep during the NR 1 & 2 sleep stages. Mr. Wakefield is prescribed amitriptyline to help with not only his back pain but also his sleep quality. His sleep medicine doctor describes the options he has for treatment of his moderate obstructive sleep apnea. He is to be scheduled with other members of the sleep medicine team, including a sleep technician to start a CPAP device trial, an ENT surgeon to evaluate his nasal congestion, and a sleep medicine dentist to evaluate for the use of a dental sleep apnea appliance.
Defining Severity of OSA
- Length of time in apnea events (measured via Apnea-Hypopnea Index (AHI))
- Percentage decrease in oxygen desaturation (oxygen nadir is the lowest desat measurement. Time desat is under 90% is a concern)
- Apnea-Hypopnea Index (AHI):
- Mild OSA= 5-15 events/hour
- Moderate OSA= 15-30 events/hour
- Severe OSA= >30 events/hour
- An apneic event is defined as: airflow decrease ≥ 90% baseline (no airflow) for ≥ 10 sec
- A hypopneic event is: airflow decrease ≥ 30% from baseline for ≥ 10 sec AND SpO2 desat ≥ 4%
- The RDI ( respiratory distress index) refers to all sleep disruptions including those that do not qualify as apneas or hypo-apneas, such as a RERA (respiratory effort-related arousal)
Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients Portable Monitoring Task Force of the American Academy of Sleep Medicine
The Portable Monitoring (PM) Task Force answered 4 questions.
- What are appropriate indications for PM?
- What types of PM should be used?
- How should PM data acquisition, analysis, and interpretation be performed?
- What is the proper application of PM results?
The 1994 review divided PM into 4 types.
- Type 1: full attended polysomnography (≥ 7 channels) in a laboratory setting
- Type 2: full unattended polysomnography (≥ 7 channels)
- Type 3: limited channel devices (usually using 4–7 channels)
- Type 4: 1 or 2 channels usually using oximetry as 1 of the parameters
Treatment of Obstructive Sleep Apnea (OSA)
- CPAP(continuous positive airway pressure) or Bi-PAP (on-demand pressure during inhalation only )
- Soft palate surgery, nasal surgery, orthognathic (jaw) surgery
- Mandibular advancement dental devices (MAD)
- Tongue retaining devices
- Oropharyngeal exercises
- Hypoglossal nerve stimulation
CPAP vs. MAD
- CPAP is the gold standard for treatment of OSA but:
- CPAP compliance is poor
- MADs are first line treatment for mild to moderate OSA and for when a severe OSA patient cannot tolerate the use of the CPAP
- MADs work best in supine positional OSA
- Medicare recommends an adjustable OSA MAD device but non-adjustable devices can be effective.
MAD = Mandibular advancement device
The Bad News About CPAP
Reasons Why CPAP is Not Used
- Appearance, vanity
- Ill-fitting mask
- Nasal congestion
- Nasal irritation and dryness
- Claustrophobia
- Difficulty falling back to sleep
- Inconvenience
Compliance in CPAP Use
- Verbal - 50-70% compliance
- Using Sensor in CPAP to measure compliance - 4.9 hours of use
- % of users who meet standard of 7 hrs/night, 5 of 7 nights - 6%
Indications for Use of Oral Appliance Therapy
- Primary / heavy snoring
- Mild / moderate OSA
- Poor tolerance for nasal CPAP
- Failure of UPPP (soft palate surgery)
- Use during travel
- In combination with nasal CPAP
Obstructive Sleep Apnea (OSA) Surgical Treatment Options
- Tonsillectomy
- Septoplasty
- UPPP
- Maxillomandibular advancement (MMA)
- Tracheotomy
- Gastric bypass
Surgical Treatments
- Tonsillectomy: removal of tonsils in patients with large tonsils
- Septoplasty: repair of deviated nasal septum
- Uvulopalatopharyngoplasty (UPPP): removal of the uvula, part of soft palate, and pharyngeal tissues (including tonsils if present); requires general anesthesia and hospital stay; most common and successful surgery
- Laser Assisted Uvuloplasty (LAUP): laser is used in office setting under local anesthetic to vaporize uvula and part of soft palate during several sessions; useful for snoring but not yet recommended for OSA
- Radiofrequency tissue ablation (RFTA) "somnoplasty": used to shrink oropharyngeal tissues
- Maxillomandibular advancement (MMA) – permanent forward repositioning of the upper/lower jaws
- Tracheostomy: direct opening in trachea, last resort but highly effective
- Gastric bypass: consider in morbidly obese patients
Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015
- Methods: The American Academy of Sleep Medicine (AASM) and American Academy of Dental Sleep Medicine (AADSM) commissioned a seven-member task force.
- A systematic review of the literature was performed and a modified Grading of Recommendations Assessment,
- Development, and Evaluation (GRADE) process was used to assess the quality of evidence.
- The task force developed recommendations for treating OSA
- The AASM’s recommendations for the use of oral appliances to treat sleep apnea are presented (slide 44-45) as standards that should be met and guidelines that must be considered
- The reader should understand that there are many dental appliances on the market that can be used to treat OSA. Over 100 have FDA approval.
- The examples of typical oral appliances presented here are used to show the different types of appliances only and do not represent should not be considered endorsements for their use
Recommendations for Oral Appliance Therapy, AASM 2015 (American Academy of Sleep Medicine)
- We recommend that sleep physicians prescribe oral appliances, rather than no therapy, for adult patients who request treatment of primary snoring (without obstructive sleep apnea). (STANDARD)
- When oral appliance therapy is prescribed by a sleep physician for an adult patient with obstructive sleep apnea, we suggest that a qualified dentist use a custom, titratable appliance over non-custom oral devices. (GUIDELINE)
- We recommend that sleep physicians consider prescription of oral appliances, rather than no treatment, for adult patients with obstructive sleep apnea who are intolerant of CPAP therapy or prefer alternate therapy. (STANDARD)
- We suggest that qualified dentists provide oversight—rather than no follow-up—of oral appliance therapy in adult patients with obstructive sleep apnea, to survey for dental-related side effects or occlusal changes and reduce their incidence. (GUIDELINE)
- We suggest that sleep physicians conduct follow-up sleep testing to improve or confirm treatment efficacy, rather than conduct follow-up without sleep testing, for patients fitted with oral appliances. (GUIDELINE)
-
We suggest that sleep physicians and qualified dentists instruct adult patients treated with oral appliances for obstructive sleep
apnea to return for periodic office visits—as opposed to no follow-up—with a qualified dentist and a sleep physician. (GUIDELINE)
AADSM: Evaluation of Sleep Disordered Breathing (SDB) and Treatment with Appliance Therapy
The Dentist must be qualified in:
- Obstructive Sleep Apnea (OSA) diagnosis
- Obstructive Sleep Apnea (OSA) effects on medical systems
- Interpretation of sleep studies
- TMD evaluations
- Interaction with other sleep specialists
- Treatment of OSA with oral appliances
Appliances
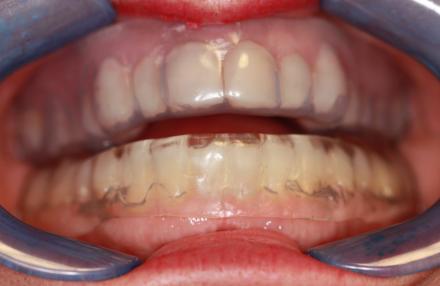
Fixed Appliance
This is a less expensive fixed appliance that is indicated for a patient without jaw pain symptoms who has the ability for good protrusion (moving jaw forward) and mild-moderate Obstructive Sleep Apnea (OSA).
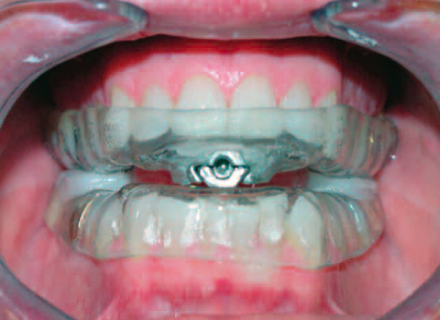
Adjustable TAP Device
This is an adjustable TAP device, which can be advanced to hold the mandible forward. Acrylic posterior stops were utilized in this case to stabilize the appliance as the patient clenched (bruxed) his teeth at night (see arrows).
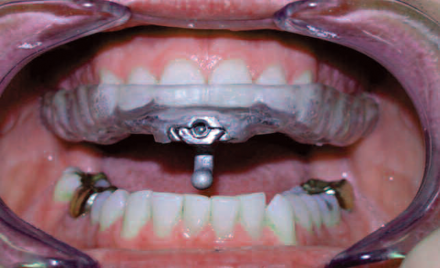
This is the upper half of an adjustable TAP device showing the hinge, which can be advanced to hold the mandible forward. It hooks onto a bar or plate on the lower half (see previous slide) preventing the mandible from moving posteriorly.
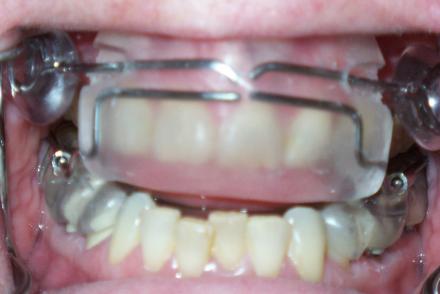
OASYS
OASYS- this an adjustable appliance that moves the mandible forward, but also has tabs (arrows) to spread the nares and also can be adjusted to promote a tongue lifting.

OASYS: this is a very adaptable appliance that can be utilized, given that the upper half is limited to only cover the facial surface of the maxillary anterior teeth, in patients with gagging issues or who have difficulty wearing oral appliances.
SomnoMedDevice
Open mouth view: The SomnoMed device is a popular adjustable appliance. The wedge on the lower half (see large blue arrow) contacts a ramp ( ) on the maxillary half that promotes the jaw to close in a forward position. The ramp position can advance the mandible forward when the patient closes his jaw.
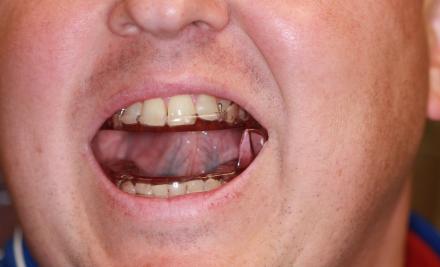
Closed mouth view: The wedge on the lower half (see large blue arrow) contacts a ramp (small blue arrow) on the maxillary half that promotes the jaw to close in a forward position. The ramp position can be advanced to protrude the mandible when the patient closes his jaw.
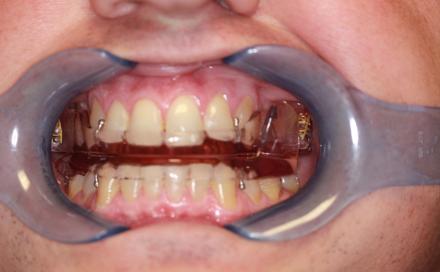
Devices that do NOT Protrude the Mandible
TRD - tongue retaining device
TLD - tongue locking device
TPE - tongue positioner and exerciser
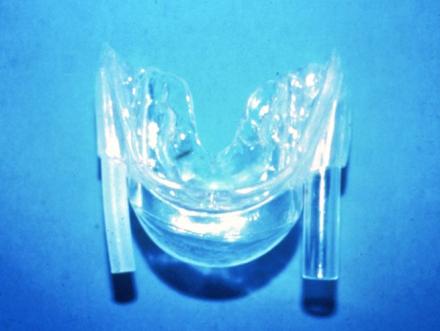
To use this device, the patient sticks his/her tongue into the bulb (arrow), which keeps the tongue forward without moving the jaw forward. The tubes adjacent to the bulb that allow the patient to breath through his/her mouth are optional, as the intent is to promote the patient to breath through the nose. Such devices have been helpful in controlling snoring, but their effectiveness for apnea is guarded.
Sleep Apnea: Take-Home Messages
- AHI and how to calculate it
- How to decide severity
- Indications for the OSA MAD device
- Contraindications: TMD – rule out
- % of occlusal changes and how to prevent them
Poor candidate for MAD?
- Periodontitis; especially anterior teeth
- Multiple missing teeth
- Severe Bruxism
- Chronic TMD
- Non-responder
Sleep Apnea Symptoms and Risk of Temporomandibular Disorder: OPPERA Cohort
V.E. Miller and G.D. Slade A.E. Sanders, G.K. Essick, R. Fillingim, C. Knott, R. Ohrbach, J.D. Greenspan, L. Diatchenko, W. Maixner, R. Dubner, E. Bair,
OPPERA
The Orofacial Pain Prospective Evaluation and Risk Assessment study (OPPERA) is a prospective cohort study designed to investigate the etiology of first-onset TMD. Its detailed methodology is described elsewhere (Slade et al., 2011). Briefly, from 2002 to 2004, 3,263 adults were recruited by community-wide advertising at 4 US study sites: Baltimore, Maryland; Buffalo, New York; Chapel Hill, North Carolina; and Gainesville, Florida. Eligibility criteria.
Hypothesis
The authors tested the hypothesis that obstructive sleep apnea (OSA) signs/symptoms are associated with the occurrence of temporomandibular disorder (TMD), using the OPPERA prospective cohort study of adults aged 18 to 44 years at enrollment (n = 2,604) and the OPPERA case-control study of chronic TMD (n = 1,716).
Abstract
The authors tested the hypothesis that obstructive sleep apnea (OSA) signs/symptoms are associated with the occurrence of temporomandibular disorder (TMD), using the OPPERA prospective cohort study of adults aged 18 to 44 years at enrollment (n = 2,604) and the OPPERA case-control study of chronic TMD (n = 1,716). In both the OPPERA cohort and case-control studies, TMD was examiner determined according to established research diagnostic criteria. People were considered to have high likelihood of OSA if they reported a history of sleep apnea or ≥ 2 hallmarks of OSA: loud snoring, daytime sleepiness, witnessed apnea, and hypertension. Cox proportional hazards regression estimated hazard ratios (HRs) and 95% confidence limits (CL) for first-onset TMD. Logistic regression estimated odds ratios (OR) and 95% CL for chronic TMD. In the cohort, 248 individuals developed first-onset TMD during the median 2.8- year follow-up. High likelihood of OSA was associated with greater incidence of first-onset TMD (adjusted HR = 1.73; 95% CL, 1.14, 2.62). In the case-control (adjusted HR = 1.73; 95% CL, 1.14, 2.62). In the case-control study, high likelihood of OSA was associated with higher odds of chronic TMD (adjusted OR = 3.63; 95% CL, 2.03, 6.52). Both studies supported a significant association of OSA symptoms and TMD, with prospective cohort evidence finding that OSA symptoms preceded first-onset TMD . were: age 18 to 44 yrs, good health, no history of facial injury or surgery, no significant symptoms of TMD pain, no previous diagnosis of TMD, and an absence of TMD myalgia and TMD arthralgia on clinical examination. The target sample size of 3,200 was expected to yield 196 cases of first-onset TMD, assuming 30% loss to follow-up, sufficient to address study aims regarding the incidence and etiology of TMD. Once enrolled, participants completed quarterly screening questionnaires to monitor the development of TMD symptoms. Symptomatic participants were recalled for examination to establish a definitive classification of incident TMD.
A case-control study of chronic TMD was embedded in OPPERA at baseline. Chronic cases were adults aged 18 to 44 yrs, with TMD symptomatic for at least 6 mos (n = 182), recruited by community-wide advertising at the same study sites. Controls were a random sample of enrollees in the cohort study (n = 1,534). All cohort and case-control participants completed health-history questionnaires and a three-hour clinical assessment that included physical examination and assessment of autonomic function. OPPERA’s unique hybrid design permitted rigorous comparison of chronic TMD vs. incident first-onset TMD phenotypes under standardized conditions.
TMD Classification
All participants were clinically examined according to Research Diagnostic Criteria for TMD (RDC/TMD) (Dworkin and LeResche, 1992) adapted for OPPERA. TMD cases met 2 criteria: (1) ≥ 5 days/ month of pain in masticatory structures, confirmed by examiner; and (2) examiner findings of arthralgia [i.e., pain in temporomandibular joint(s) during jaw maneuver or digital palpation] and/or myalgia (i.e., pain during jaw maneuver or digital palpation in ≥ 3 of 8 muscle groups based on bilateral assessment of temporalis, masseter, lateral pterygoid, and submandibular muscles).
Signs/Symptoms of Obstructive Sleep Apnea.
From the Pittsburgh Sleep Quality Index (PSQI), we extracted responses to 3 questions that asked about loud snoring, trouble staying awake, and witnessed apnea. From the medical history, we extracted hypertension information. Together, these 4 hallmarks of OSA comprise the 4-item OSA screening questionnaire called STOP that was validated against polysomnography (Chung et al., 2008). STOP was developed to identify individuals likely to have OSA, so that special precautions could be taken during/after general anesthesia. Affirmative responses to ≥ 2 of these questions indicated high likelihood for OSA. We adopted this scoring convention, and, consistent with the
Earlier studies of OSA and TMD, conducted in unrepresentative clinic samples without a comparison group, were nonetheless informative because of the inclusion of in-laboratory polysomnographic recordings. Among 87 adults diagnosed by polysomnography as having mild or moderate OSA, 32 (36.8%) had TMD determined by RDC/ TMD criteria (Cunali et al., 2009). In another study, 11 adults (28%) of 53 with RDC/TMD-based myofascial pain were diagnosed by polysomnography as having OSA (Smith et al., 2009). OPPERA’s population-based studies extend these case series reports by quantifying the strength of association and demonstrating that OSA symptoms are associated with both first-onset TMD and chronic TMD. This study’s findings of an association between OSA symptoms and TMD contribute to a growing body of evidence documenting a relationship
between sleep-disordered breathing and other idiopathic pain conditions, including fibromyalgia (Moldofsky et al., 1975), irritable bowel syndrome (Rotem et al., 2003), and headache (Rains and Poceta, 2012).
One mechanism by which SDB may contribute to pain over time is through the effect of central sensitization and pain amplification in decreasing function in pain inhibitory systems (Smith et al., 2007), for example, via baroreceptor pathways. Experimental inspiratory resistance reduces baroreceptor reflex sensitivity in humans, and impaired baroreceptor reflex sensitivity is implicated in TMD (Maixner et al., 1997). It is also possible that the increased stimulation of the sympathetic nervous system observed in OSA underlies an increased prevalence of TMD: Individuals who are genetically predisposed to an increased sensitivity to catecholamines are at increased risk of developing first-onset TMD (Diatchenko et al., 2005). Third, although a causal association remains controversial (Manfredini et al., 2012), bruxism—which is more common in people with OSA than in those without OSA (Ohayon et al., 2001)—may be implicated in TMD development.
Strengths and Limitations
Strengths of the study include the prospective cohort design, which established the presence of OSA signs/ symptoms prior to first-onset TMD, the rigorous OPPERA protocols, and the rich OPPERA dataset. Both the cohort and case-control studies were large and population-based, with statistical power to detect small effects. Multiple study sites and community recruitment optimized the diversity and representativeness of the study population to improve the generalizability of findings. Although causality cannot be inferred from observational data alone, the prospective cohort findings imply that OSA contributes to the onset of painful TMD symptoms. Our adjustment for subjective sleep quality in analytic models constitutes “over-adjustment”, because poor sleep quality is a consequence both of pain and of the fragmented sleep evoked by sleep-disordered breathing. Over-adjustment tends to bias results toward the null. Instead, our purpose in adjusting for subjective sleep quality was to demonstrate that our main exposure– OSA symptomatology–was independently associated with TMD rather than merely a proxy of sleep quality. Even with this excessively conservative approach, OSA symptoms remained a significant factor.
The gold-standard for OSA diagnosis is laboratory polysomnography. The STOP has sensitivity of 0.66 and specificity of 0.60 to classify OSA in adults without history of sleep disorders (Chung et al., 2008) and inevitably misclassifies OSA risk. However, since there is no reason for misclassification to differ between TMD cases and non-cases, resulting bias will shift estimates of association conservatively toward the null.
The strength of association between OSA signs/symptoms and TMD may be underestimated in this young OPPERA population. It is likely that the relationship between OSA and TMD is stronger among older adults, especially those with co-morbid conditions, including obesity.
Missing data arising from loss to follow-up poses a major threat to validity in prospective cohort studies. We did not impute values for participants lost to follow-up or data missing through non-report. This decision was justified because our multiple imputation techniques and sensitivity analyses showed that doing so did not appreciably change the incidence rate (Bair et al., manuscript under review (Article in AAOP meetings 2014 file).
Shimstock
Use of shimstock to monitor occlusion and check for bite changes.
Shimstock is .05 “ thick and allows an accurate measure of which teeth contact.
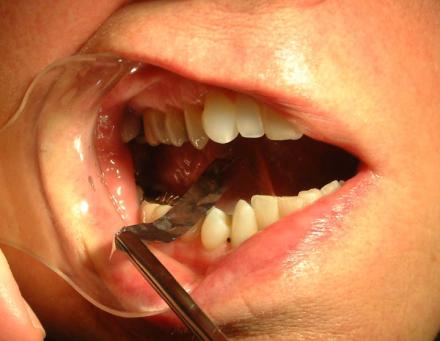
10-12 % of patients using MAD for the treatment of OSA can develop bite changes. These develop not because the teeth move but because the arc of closure of the mandible moves anteriorly from the repeated anterior position of mandible during the night with appliance therapy. The anterior movement of the arc of closure promotes the anterior teeth to contact first and creating a posterior teeth open bite (the patient cannot get his posterior teeth (molars and bicuspids) to contact)). The use of shimstock allows early detection of bite changes before the patient is aware that a posterior open bite is developing.
Swallowing exercises in the morning and use of a template that duplicates the original position of the mandible (the patient closes into it) can be used to prevent arc of closure bite changes. Tooth contacts should be recorded before appliance therapy begins to document the patient’s occlusal contacts. These contacts are then monitored at each of the patient’s follow-up appointments.
Leaf Gauge
Dental Appliance Protocol
- Pulmonary medicine obtains one night study to establish diagnosis
- Dental consultation
- Second sleep study with appliance if subjective improvement
- CPAP titration if improvement and indicated
Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea
Mandibular advancement splints (MAS) are an effective treatment for obstructive sleep apnea (OSA); however, therapeutic response is variable. Younger age, female gender, less obesity, and milder and supine-dependent OSA have variably been associated with treatment success in relatively small samples.
This study’s objective was to utilize a large cohort of MAS treated patients (1) to compare efficacy across patients with different phenotypes of OSA and (2) to assess demographic, anthropometric, and polysomnography variables as treatment response predictors.
Study Objectives
Mandibular advancement splints (MAS) are an effective treatment for obstructive sleep apnea (OSA); however, therapeutic response is variable. Younger age, female gender, less obesity, and milder and supine-dependent OSA have variably been associated with treatment success in relatively small samples. Our objective was to utilize a large cohort of MAS treated patients (1) to compare efficacy across patients with different phenotypes of OSA and (2) to assess demographic, anthropometric, and polysomnography variables as treatment response predictors.
Methods
Retrospective analysis of MAS-treated patients participating in clinical trials in sleep centers in Sydney, Australia between years 2000–2013. All studies used equivalent customized two-piece MAS devices and treatment protocols. Treatment response was defined as (1) apnea-hypopnea index (AHI) < 5/h, (2) AHI < 10/h and ≥ 50% reduction, and (3) ≥ 50% AHI reduction.
Results
A total of 425 patients (109 female) were included (age 51.2 ± 10.9 years, BMI 29.2 ± 5.0 kg/m2). MAS reduced AHI by 50.3% ± 50.7% across the group. Supine-predominant OSA patients had lower treatment response rates than non-positional OSA (e.g., 36% vs. 59% for AHI < 10/h). REM-predominant OSA showed a lower response rate than either NREM or non-stage dependent OSA. In prediction modeling, age, baseline AHI, and anthropometric variables were predictive of MAS treatment outcome but not OSA phenotype. Gender was not associated with treatment outcome.
Conclusions
Lower MAS treatment response rates were observed in supine and REM sleep. In a large sample, we confirm that demographic, anthropometric, and polysomnographic data only weakly inform about MAS efficacy, supporting the need for alternative objective prediction methods to reliably select patients for MAS treatment.
Reference
Sutherland K. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015 Aug 15; 11(8): 861–868. Published online 2015 Aug 15. doi: 10.5664/jcsm.4934
Mr. Wakefield Summary of Treatment for Obstructive Sleep Apnea (OSA)
- Mr. Wakefield sleep onset and duration improve with his treatment of depression and anxiety
- He is diagnosed with moderate obstructive sleep apnea with an AHI of 18 and an oxygen desaturation nadir of 84%
- Although during his sleep study a CPAP device controls his apneic events he decides to undergo treatment with a dental sleep apnea device.
- He is treated with an elastomeric over thermoplastic fixed device which he can wear nightly and he subjectively states that he feels more rested when he awakens and has more energy during the day.
- A follow-up sleep study shows an AHI of 4.8 and improved oxygen desaturation levels.

