After completing a pain assessment, the dentist summarizes the visit this way:
Chart Notes
The patient reports being most aware of the symptoms during and shortly after oral function such as speaking and eating. However, baseline pain is continually present and has been increasing in recent weeks. The patient is not aware of teeth grinding while sleeping.
Types of Oral and Facial Pain
This figure illustrates the defined region of Oral and Facial pain.
Oral and Facial pain can be described in terms of location, quality, or duration (acute, recurrent vs persistent or chronic)
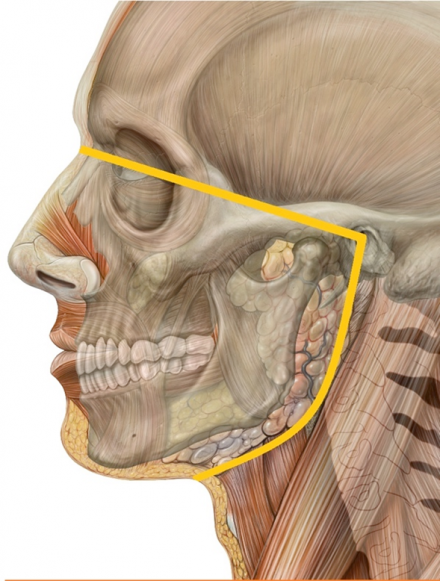
*Image used by permission from https://en.wikipedia.org/wiki/Orofacial_pain#/media/File:Orofacial_pain_Lateral_head_skull.jpg
Nociceptive Pain
Originates from musculoskeletal or visceral structures
Periodontal pain, muscle pain, temporomandibular joint disorder, pulpitis
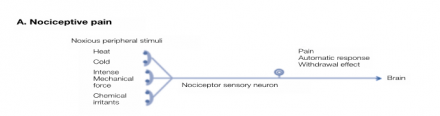
Nociceptive pain is usually well-localized, described as aching or throbbing, and exacerbated by functional provocation.
Inflammatory Pain
Immune cells release substances that activate neurons and increase sensitivity
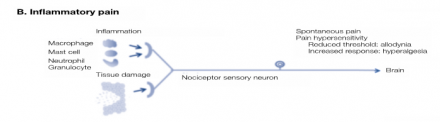
Inflammatory pain is nociceptive but results in ongoing pain and tenderness or hypersensibility. Allodynia and hyperalgesia may result from the chemical inflammatory mediators released into the injured tissues and increase the responsiveness or nociceptor endings. This is known as peripheral sensitization.
Neuropathic Pain
Pain from a disease or lesion of the somatosensory nervous system
Trigeminal neuralgia, glossopharyngeal neuralgia, burning mouth syndrome, surgical injury
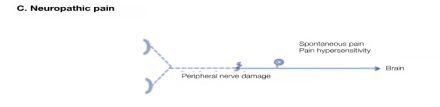
Neuropathic pain is usually described as sharp, shooting, electrical shock or burning pain. Allodynia and/or hyperalgesia and/or dysesthesia are commonly reported with pain and may be diagnostic.
Functional Pain
Increased perception of pain without ongoing peripheral disease or injury signals
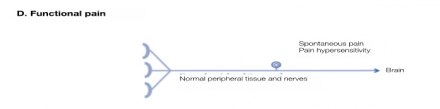
Functional Pain is real pain without identifiable pathology.
The Case Continues
Questions to Consider:
- Patient was seen by dentist 2 months ago and diagnosed with TMD (TMJ Disorder) or stress.
- Given this differential diagnosis, how would you treat this patient's pain?
- What additional members of the healthcare team would you consult?
- When would you advise the patient to follow-up with you?
Additional Information
Patient was prescribed a nightguard which is used every night while sleeping. The clicking persisted, but was improved. However, jaw pain and headaches increased in frequency, and mobility of mouth actually decreased.
The patient's parents became increasingly concerned as they observed oral intake significantly decrease during winter break. The patient and family sought further assessment and treatment from you.
What else do you need to do to obtain a thorough assessment of the patient?
- Self-report pain assessment
- Functional assessment and exam
- Physical exam
- Pain history
- Family history
- MRI- warning when selected
- Other assessments? Ask your team
Chart
Weight
60 kg
Height
160 cm
VS
Afebrile, HR 70, RR 24, BP 110/66
Pain
Intensity
4 of 10
Location
Head, neck, left eye and ear.
Quality
Pain described as a fullness and pressure of forehead, left eye, and jaw
Timing
Pain is constant, with unpredictable and short bursts of increased intensity and shooting quality
Aggravating
Pain is increased with jaw movement
Alleviating
Unable to identify any treatment that has provided prolonged pain relief
General
Appears well-nourished, appropriate size for height, well groomed
Eyes
PERRL, EOM intact, No indication of increased ocular pressure or intracranial pressure.
HET
Facial musculature was negative to palpation, tenderness with light pressure to TMJ, +restricted movement < 15mm maximum interocclusal opening, mild uvula deviation, tonsils obscured by edema
Neck
No swelling of neck, but tender lymphadenopathy left >right
Respiratory
No pertinent findings on exam
MS
No pertinent findings on exam
Cardiovascular
No pertinent findings on exam
Neuro
No pertinent findings on exam
GI
No pertinent findings on exam
Skin
No rash, no bruises
GU
No pertinent findings on exam
Psych
No pertinent findings on exam
Oral and Facial Pain Assessment: Your Patient
Oral and Facial Innervation
Like the skin in other parts of the body, the skin of the face and scalp are innervated with heavily myelinated low-threshold mechanoreceptive afferents, Aβ fibers, thinly myelinated afferents, Aδ fibers, and unmyelinated C fibers. Many C fibers are silent and can only be activated when sensitized by inflammation. The skin over the parotid and lower ear lobe are supplied by C2 and C3 (not the trigeminal nerve).
Teeth have intrinsic and extrinsic innervation. The intrinsic innervation associated with tooth pulp report damage, such as caries or a broken tooth, whereas the periodontium and gingival soft tissues are extrinsically innervated and have a low-threshold to touch.
Less is known about the intrinsic innervation of the Oral and Facial bones. Tumors within the mandibular bone tend not to be painful unless the bone is breached and the tumor exerts pressure on nearby innervated tissues.
The brain parenchyma, including the retina, do not receive any nociceptive innervation.
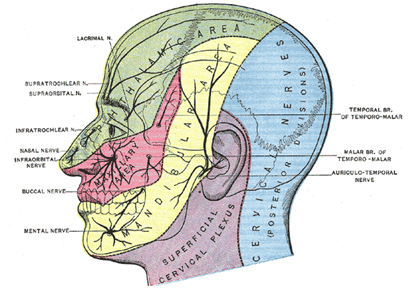
Image used by permission from https://commons.wikimedia.org/wiki/File:Gray784.png
Sensory Pathways
Although sensory pathways are often depicted as chains of individual neurons connected in series, this is an oversimplification. Sensory information is processed and modified at each level in the chain by interneurons and input from other areas of the nervous system.
Oral and Facial pain begins as a neural impulse (e.g., action potential) generated by peripheral neurons, also known as primary afferent neurons, comprising the trigeminal nerve (cranial nerve V) innervating the temporomandibular joint (TMJ). This impulse travels from the TMJ along the trigeminal nerve to the trigeminal root ganglion. First order primary afferents synapse onto secondary neurons in the spinal trigeminal nucleus located in the medulla
There are several anatomic and functional differences in the primary afferent neurons of the trigeminal ganglia that distinguish them from neurons of the spinal dorsal root ganglia. First, there is a higher ratio of myelinated to non-myelinated afferent fibers.
Second, MesV (Mesencephalic Nucleus (located in the brain stem) ) contains cell bodies of primary afferent proprioceptors, or afferents. These innervate the jaw closing muscles.
The axons of the muscle and periodontal afferents of MesV neurons travel in the motor rather than the sensory root of the trigeminal nerve (cranial nerve V).
Oral and Facial innervation is also provided by the glosso- pharyngeal and vagus nerves; but the three branches (V1 ophthalmic, V2 maxillary, V3 and mandibular nerves) of the trigeminal nerve innervate most head tissues except the back of the skull.
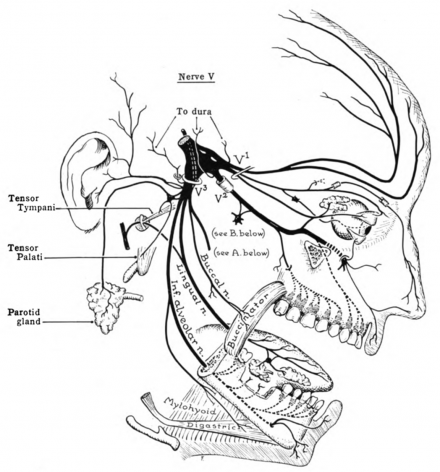
*Image used by permission from https://en.wikipedia.org/wiki/Trigeminal_nerve
Electrical to Chemical Communication: Synaptic Transmission
The transition from first order primary afferents to secondary neurons within the spinal trigeminal nucleus requires the impulse change from an electrical signal to a chemical one.
Chemicals called neurotransmitters are released from the sensory terminals of presynaptic neurons into the synaptic cleft. Neurotransmitters traverse the synapse where they bind to the appropriate receptors on the postsynaptic neuron and cause the generation of a postsynaptic action potential.
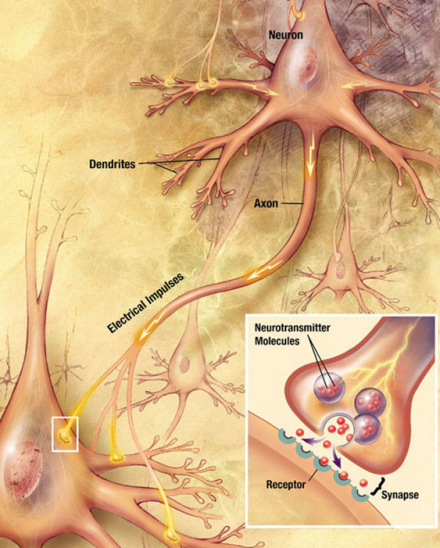
*Image used by permission from https://en.wikipedia.org/wiki/Chemical_synapse
Electrical to Chemical Communication: Synaptic Transmission
There are numerous neurotransmitters that can be released from neurons, but the primary transmitters released from the trigeminal nerve include calcitonin gene-related peptide (CGRP), substance P (SP), serotonin (5-HT), glutamate, adenosine triphosphate (ATP).
The binding of these neurotransmitters to their respective receptors is the opening of associated ion channels that allow Na+, K+, Ca2+ to enter or exit the cell.
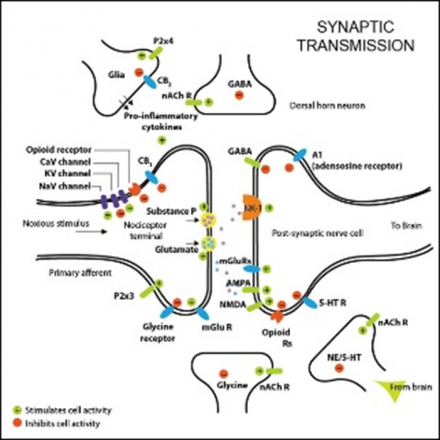
*Image used by permission from https://en.wikipedia.org/wiki/Neurotransmitter
The net effect of this neurotransmitter activity and ion flow in the transmission of pain-related action potentials is an increase in firing of neurons within the trigmeninal pathway. Increased firing rates result from the decrease in threshold required for neurons to fire action potentials, increased neuoronal sensitivity, and increased spontaneous activity.
Neurotransmitters do not remain in the synaptic cleft indefinitely. They can diffuse out of the cleft and bind to transporters on the presynaptic neuron that allow them to enter the cell and be recycled. Others are metabolized in the cleft by enzymes.
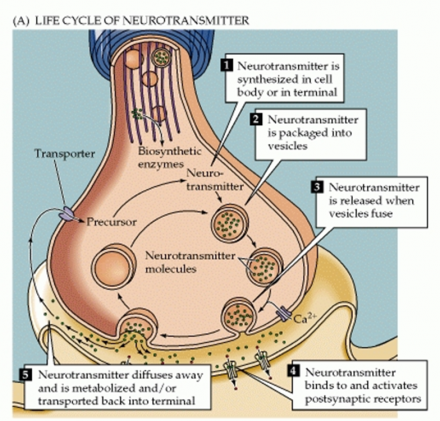
*Image used by permission from https://en.wikipedia.org/wiki/Neurotransmitter
Rise of Oral and Facial Pain: Trigeminal Pathway
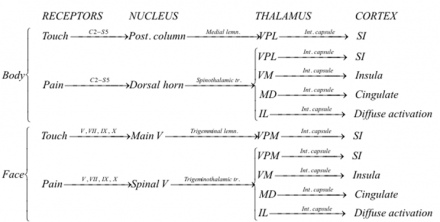
The impulse leaves the trigeminal ganglion and travels to the spinal trigeminal nucleus where the first order primary afferents synapse with the second order neurons in the trigeminal nucleus.
The impulse crosses over the midline and travels up contralateral tracts to the thalamus. The secondary neurons in each pathway decussate (cross the spinal cord or brainstem) because the spinal cord develops in segments. Decussated fibers later reach and connect these segments with the higher centers, synapsing onto third order neurons that relay information to various cortical destinations.
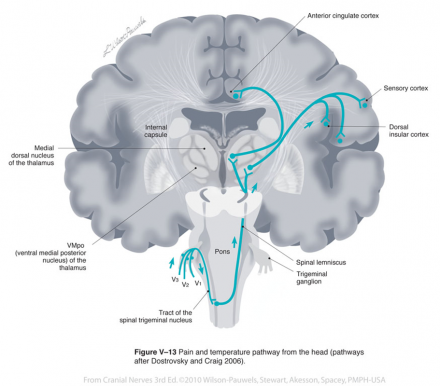
All sensory information is sent to specific nuclei in the thalamus. Thalamic nuclei, in turn, send information to specific areas in the cerebral cortex.
Animation: https://en.wikipedia.org/wiki/Thalamus
Pain-temperature information from the face is carried to the thalamus by the anterior trigeminothalamic tract.
Pathways for the face and body merge in the brainstem, and touch-position and pain-temperature sensory maps of the entire body are projected onto the thalamus. From the thalamus, information is projected onto the cerebral cortex.
Unlike touch-position information, however, pain-temperature information is also sent to other thalamic nuclei and projected onto additional areas of the cerebral cortex.
Some pain-temperature fibers are sent to the medial dorsal thalamic nucleus (MD), which projects to the anterior cingulate cortex. Other fibers are sent to the ventromedial (VM) nucleus of the thalamus, which projects to the insular cortex.
Finally, some fibers are sent to the intralaminar nucleus (IL) of the thalamus via the reticular formation. The IL projects diffusely to all parts of the cerebral cortex.
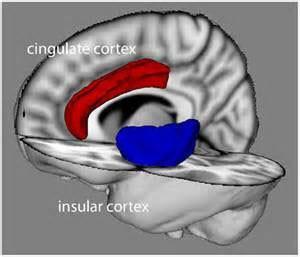
*Image used by permission from Creative Commons License: Copyright: © 2012 Seth, Suzuki and Critchley
The insular and cingulate cortices are parts of the brain which represent pain-temperature in the context of other simultaneous perceptions (sight, smell, taste, hearing and balance) and in the context of memory and emotional state.
Peripheral pain-temperature information is channeled directly to the brain at a deep level, without prior processing. Peripheral pain-temperature information also feeds directly into this system.
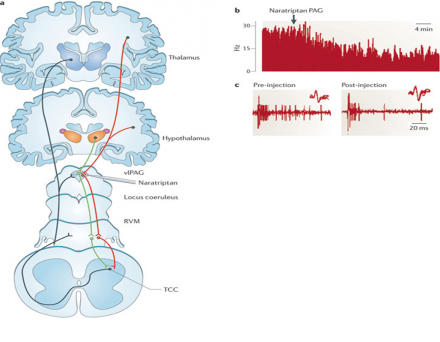
Descending Modulation of Trigeminal System
Painful information that is transmitted from the peripheral to central nervous system can be modulated to influence its eventual perception.
In particular, these signals can be dampened or enhanced from structures in the brain through processes known as descending inhibition and descending facilitation, respectively.
Incoming nociceptive signals from Oral and Facial structures are processed in the trigemino- cervical complex (TCC), and then through ascending projections to the pain processing centers in the rostral ventromedial medulla (RVM), ventrolateral periaqueductal grey (vlPAG) and thalamus.
These nociceptive signals are under descending control by 'on' and 'off' cell projections from the vlPAG, via the RVM ('on' cells are represented by green fibres, and 'off' cells are represented by red fibres).
Thus pain inhibits pain. vlPAG is also activated by both endogenous and exogenous opioids.
Functional and anatomical studies link the descending pain modulatory system from the brain stem (where the PAG and RVM reside) to a number of higher level brain areas, including: cingulofrontal regions, the amygdalae and the hypothalamus.
These connections help explain the generation of autonomic and neuroendocrine responses and the role emotions and cognition have in processing nociceptive information.
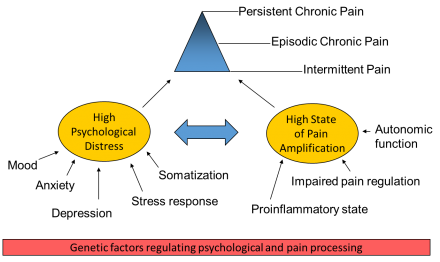
Biopsychosocial Model of Pain
When pain persists and becomes chronic or recurrent, it can exceed the resources for effective coping and the resulting disability associated with the pain. From a biological perspective, sympathetic nervous system activation tends to make pain more intense and difficult to manage. The good news is that sympathetic nervous system activation is readily modifiable (ex. watching a scary movie vs. relaxing).
Response to pain can be influenced by:
- Self-regulation
- Catastrophizing
- Somatization
- Attention and Distraction
- Support systems
- Nutrition
Non-Pharmacologic Pain Coping Strategies
Non-pharmacologic (biobehavioral) coping strategies can modify sympathetic nervous system activation to help patients cope with recurrent, persistent and chronic pain.
- Diaphragmatic (“deep” or “belly”) breathing techniques
- Relaxation techniques
- Guided imagery - self-hypnosis
- Progressive muscle relaxation
- Meditation
- Biofeedback
Additional cognitive behavioral strategies include:
Problem solving
- Use of coping statements
- Time management
- Distraction
- Journaling
- Improving sleep hygiene
- Developing a more active lifestyle

